No Data
US OptionsDetailed Quotes
BMEA241220P22500
- 0.00
- 0.000.00%
15min DelayClose Dec 13 09:30 ET
0.00High0.00Low
0.00Open0.00Pre Close0 Volume0 Open Interest22.50Strike Price0.00Turnover0.00%IV-298.94%PremiumDec 20, 2024Expiry Date16.86Intrinsic Value100Multiplier6DDays to Expiry0.00Extrinsic Value100Contract SizeAmericanOptions Type--Delta--Gamma0.34Leverage Ratio--Theta--Rho--Eff Leverage--Vega
Intraday
- 5D
- Daily
No Data
News
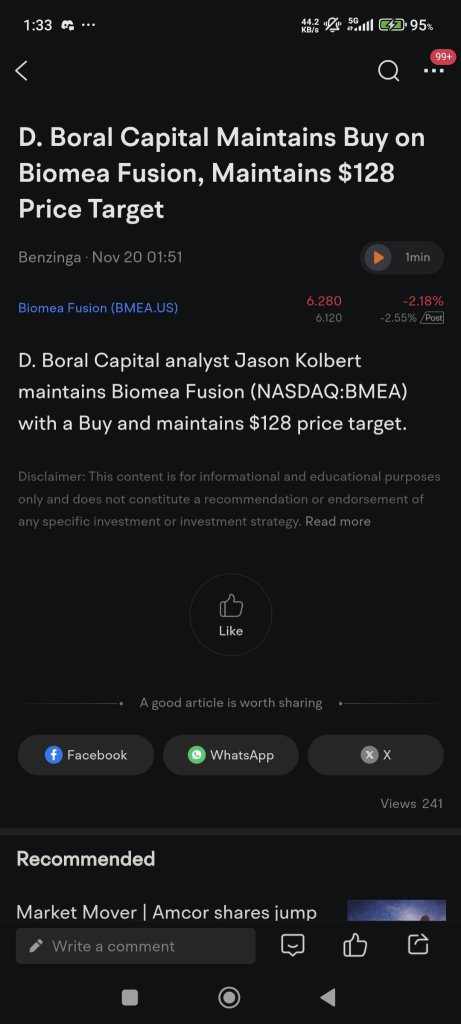
D. Boral Maintains Buy Rating, $128 Target on Biomea Fusion After Presentations
Express News | Biomea Fusion Says In Preclinical Experiments, Icovamenib Enhanced Beta Cell Function And Responsiveness Of Human Islets To GLP-1-Based Therapies
Express News | Biomea Fusion Announces Oral and Poster Presentations of Icovamenib at the 22Nd World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease (Wcirdc)
Biomea Fusion Slides As Insider Purchases Lose Another US$26k
Biomea Fusion Price Target Maintained With a $128.00/Share by D. Boral Capital
Biomea Fusion Is Maintained at Buy by D. Boral Capital
Comments
$Biomea Fusion (BMEA.US)$ Reuters· just
Biomea Fusion Announces Preliminary Data From Ongoing Covalent-103 Study of Investigational Covalent Flt3 Inhibitor Bmf-500 in Relapsed or Refractory Acute Leukemia
Biomea Fusion Announces Preliminary Data From Ongoing Covalent-103 Study of Investigational Covalent Flt3 Inhibitor Bmf-500 in Relapsed or Refractory Acute Leukemia
1
$Biomea Fusion (BMEA.US)$ wonder how they come out with such T.P?...![]()
1months from profit tanked to losses...
1months from profit tanked to losses...

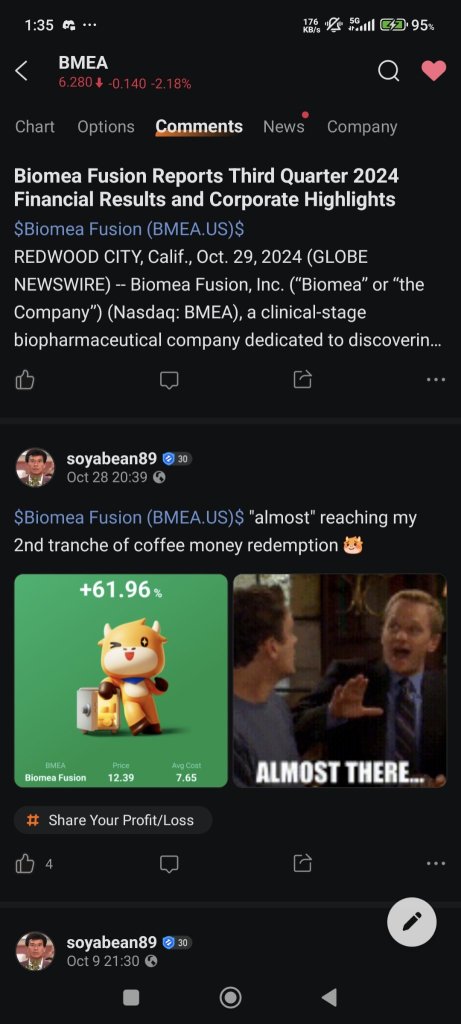
4
10
$Biomea Fusion (BMEA.US)$ Reuters· 1 min ago
Biomea Fusion Inc - Preclinical Data Shows Bmf-219 Enhanced Effectiveness of GLP-1-Based Therapies
Biomea Fusion Inc - Preclinical Data Shows Bmf-219 Enhanced Effectiveness of GLP-1-Based Therapies
1
Read more

Trytosaveabit OP : Preliminary data supports BMF-500's potential as a transformative therapy for patients with FLT3 mutated relapsed or refractory (R/R) acute leukemia
BMF-500 showed a favorable safety and tolerability profile, with no dose-limiting toxicities observed across all dose levels
Pharmacokinetic and pharmacodynamic data confirmed on-target FMS-like tyrosine kinase 3 (FLT3) inhibition, demonstrating dose-proportional activity and good compartmental penetration
Preliminary Phase I data for BMF-500 in R/R acute leukemia patients with FLT3 gene mutations having failed gilteritinib indicated clinical activity with evidence of responses, including a first complete response with incomplete hematologic recovery (CRi) and reductions in bone marrow blasts in 5 of 6 of the evaluable FLT3 mutated patients