US OptionsDetailed Quotes
BMEA241220P7500
- 2.70
- 0.000.00%
15min DelayClose Dec 13 16:00 ET
2.70High2.70Low
2.70Open2.70Pre Close1 Volume3.50K Open Interest7.50Strike Price270.00Turnover497.95%IV14.89%PremiumDec 20, 2024Expiry Date1.86Intrinsic Value100Multiplier6DDays to Expiry0.84Extrinsic Value100Contract SizeAmericanOptions Type-0.5559Delta0.1118Gamma2.09Leverage Ratio-0.1251Theta-0.0008Rho-1.16Eff Leverage0.0028Vega
Biomea Fusion Stock Discussion
$Biomea Fusion (BMEA.US)$ Reuters· just
Biomea Fusion Announces Preliminary Data From Ongoing Covalent-103 Study of Investigational Covalent Flt3 Inhibitor Bmf-500 in Relapsed or Refractory Acute Leukemia
Biomea Fusion Announces Preliminary Data From Ongoing Covalent-103 Study of Investigational Covalent Flt3 Inhibitor Bmf-500 in Relapsed or Refractory Acute Leukemia
1
$Biomea Fusion (BMEA.US)$ wonder how they come out with such T.P?...![]()
1months from profit tanked to losses...
1months from profit tanked to losses...



4
10
$Biomea Fusion (BMEA.US)$ Reuters· 1 min ago
Biomea Fusion Inc - Preclinical Data Shows Bmf-219 Enhanced Effectiveness of GLP-1-Based Therapies
Biomea Fusion Inc - Preclinical Data Shows Bmf-219 Enhanced Effectiveness of GLP-1-Based Therapies
1
$Biomea Fusion (BMEA.US)$
REDWOOD CITY, Calif., Oct. 29, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or “the Company”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to improve the lives of patients with diabetes, obesity, and genetically defined cancers, today reported third quarter 2024 financial results and corporate hig...
REDWOOD CITY, Calif., Oct. 29, 2024 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea” or “the Company”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small molecules to improve the lives of patients with diabetes, obesity, and genetically defined cancers, today reported third quarter 2024 financial results and corporate hig...


4


1
1
$Biomea Fusion (BMEA.US)$ after hours could be interesting
No comment yet

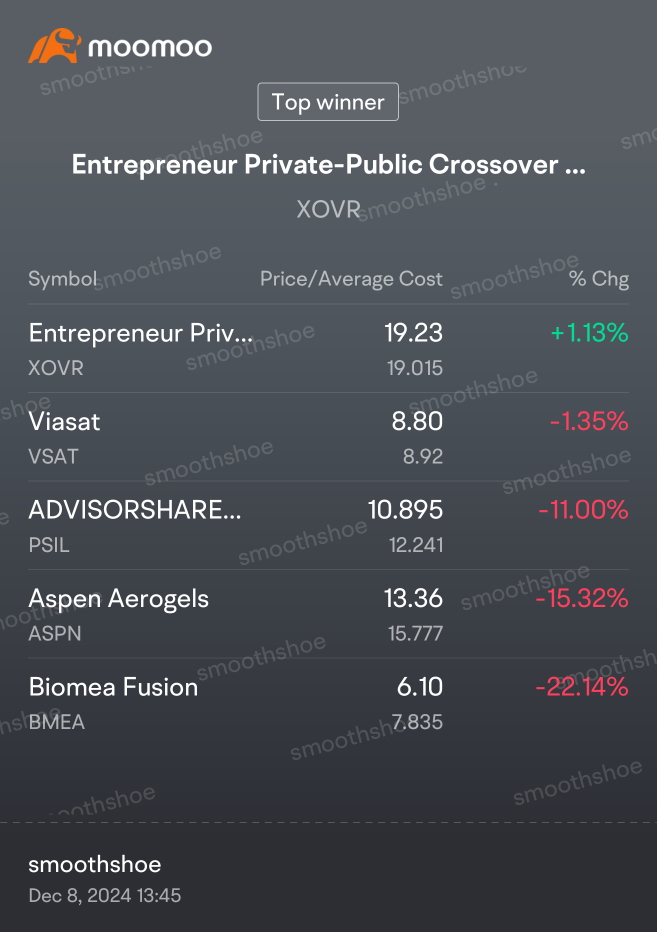



Trytosaveabit OP : Preliminary data supports BMF-500's potential as a transformative therapy for patients with FLT3 mutated relapsed or refractory (R/R) acute leukemia
BMF-500 showed a favorable safety and tolerability profile, with no dose-limiting toxicities observed across all dose levels
Pharmacokinetic and pharmacodynamic data confirmed on-target FMS-like tyrosine kinase 3 (FLT3) inhibition, demonstrating dose-proportional activity and good compartmental penetration
Preliminary Phase I data for BMF-500 in R/R acute leukemia patients with FLT3 gene mutations having failed gilteritinib indicated clinical activity with evidence of responses, including a first complete response with incomplete hematologic recovery (CRi) and reductions in bone marrow blasts in 5 of 6 of the evaluable FLT3 mutated patients