No Data
US Stock MarketDetailed Quotes
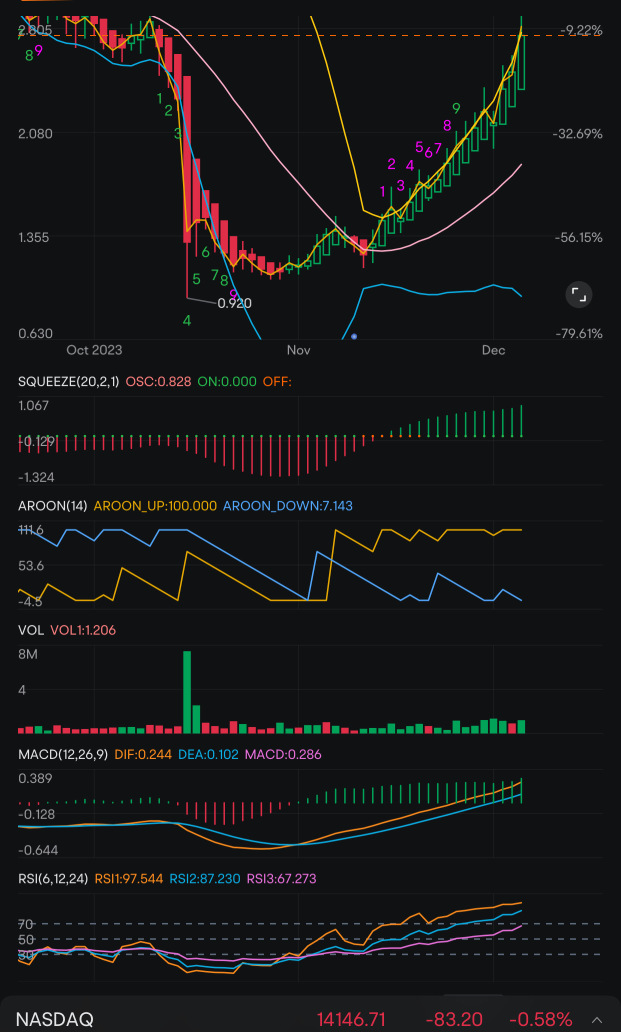
OMER Omeros
- 4.040
- -0.180-4.27%
Close Oct 31 16:00 ET
- 4.040
- 0.0000.00%
Post 20:01 ET
234.11MMarket Cap-1719P/E (TTM)
4.220High4.010Low230.24KVolume4.210Open4.220Pre Close936.29KTurnover0.42%Turnover RatioLossP/E (Static)57.95MShares5.68052wk High-1.88P/B223.70MFloat Cap1.13052wk Low--Dividend TTM55.37MShs Float30.230Historical High--Div YieldTTM4.98%Amplitude0.920Historical Low4.066Avg Price1Lot Size
Full Hours
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Omeros Receives Rare Pediatric Disease Designation for Zaltenibart From FDA
Express News | FDA Grants Rare Pediatric Disease Designation to Omeros’ Masp-3 Inhibitor Zaltenibart for Treatment of C3 Glomerulopathy
Needham Sticks to Its Hold Rating for Omeros (OMER)
Omeros: Q2 Earnings Snapshot
Express News | Omeros Q2 2024 GAAP EPS $(0.97) Beats $(1.14) Estimate
Omeros | 10-Q: Q2 2024 Earnings Report
Comments
$Omeros (OMER.US)$
FDA Grants Rare Pediatric Disease Designation to Omeros’ MASP-3 Inhibitor Zaltenibart for Treatment of C3 Glomerulopathy
Omeros announced that its MASP-3 inhibitor zaltenibart (OMS906) received rare pediatric disease designation from the FDA for treating C3 glomerulopathy (C3G), an ultra-rare renal disorder affecting children and young adults. The company plans to initiate Phase 3 trials for C3G in 2024. Zaltenibart is also being developed for paroxysmal nocturnal hemoglob...
FDA Grants Rare Pediatric Disease Designation to Omeros’ MASP-3 Inhibitor Zaltenibart for Treatment of C3 Glomerulopathy
Omeros announced that its MASP-3 inhibitor zaltenibart (OMS906) received rare pediatric disease designation from the FDA for treating C3 glomerulopathy (C3G), an ultra-rare renal disorder affecting children and young adults. The company plans to initiate Phase 3 trials for C3G in 2024. Zaltenibart is also being developed for paroxysmal nocturnal hemoglob...
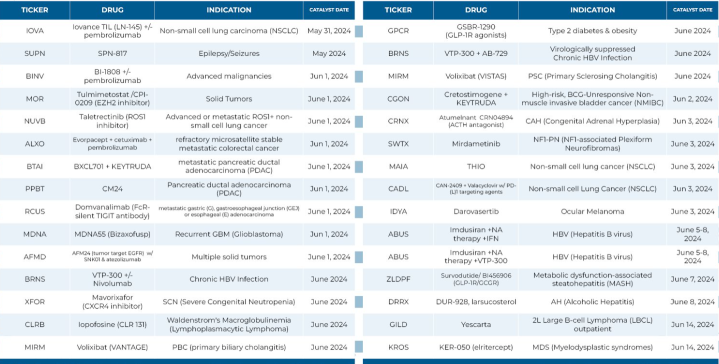
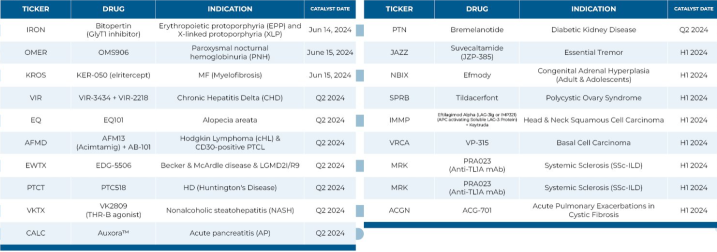
🎯 Ph 2 readouts in Q2 2024...
$Iovance Biotherapeutics (IOVA.US)$ Phase 2
$Supernus Pharmaceuticals (SUPN.US)$ Phase 2a
$BRANDES INTERNATIONAL ETF (BINV.US)$ Phase 2a
$MorphoSys (MOR.US)$ Phase 2
$Nuvation Bio (NUVB.US)$ Phase 2
$ALX Oncology (ALXO.US)$ Phase 2
$BioXcel Therapeutics (BTAI.US)$ Phase 2
$Purple Biotech (PPBT.US)$ Phase 2
$Arcus Biosciences (RCUS.US)$ Phase 2
$Medicenna Therapeutics (MDNAF.US)$ Phase 2b
$Affimed NV (AFMD.US)$ Phase 2
$Barinthus Biotherapeutics (BRNS.US)$ Phase 2b...
$Iovance Biotherapeutics (IOVA.US)$ Phase 2
$Supernus Pharmaceuticals (SUPN.US)$ Phase 2a
$BRANDES INTERNATIONAL ETF (BINV.US)$ Phase 2a
$MorphoSys (MOR.US)$ Phase 2
$Nuvation Bio (NUVB.US)$ Phase 2
$ALX Oncology (ALXO.US)$ Phase 2
$BioXcel Therapeutics (BTAI.US)$ Phase 2
$Purple Biotech (PPBT.US)$ Phase 2
$Arcus Biosciences (RCUS.US)$ Phase 2
$Medicenna Therapeutics (MDNAF.US)$ Phase 2b
$Affimed NV (AFMD.US)$ Phase 2
$Barinthus Biotherapeutics (BRNS.US)$ Phase 2b...
16
3
$Omeros (OMER.US)$ Omeros Announces Online Publication in Advance of ASH Annual Meeting Detailing Narsoplimab Treatment Under Compassionate Use of 15 Patients with Hematopoietic Stem Cell Transplant-associated Thrombotic Microangiopathy
7 MINUTES AGO, 9:05 AM EDT
7 MINUTES AGO, 9:05 AM EDT
Read more
Analysis
Price Target
No Data
Heat List
Overall
Symbol
Latest Price
% Chg
No Data

