No Data
US Stock MarketDetailed Quotes
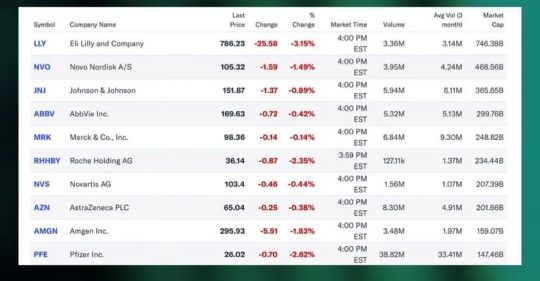
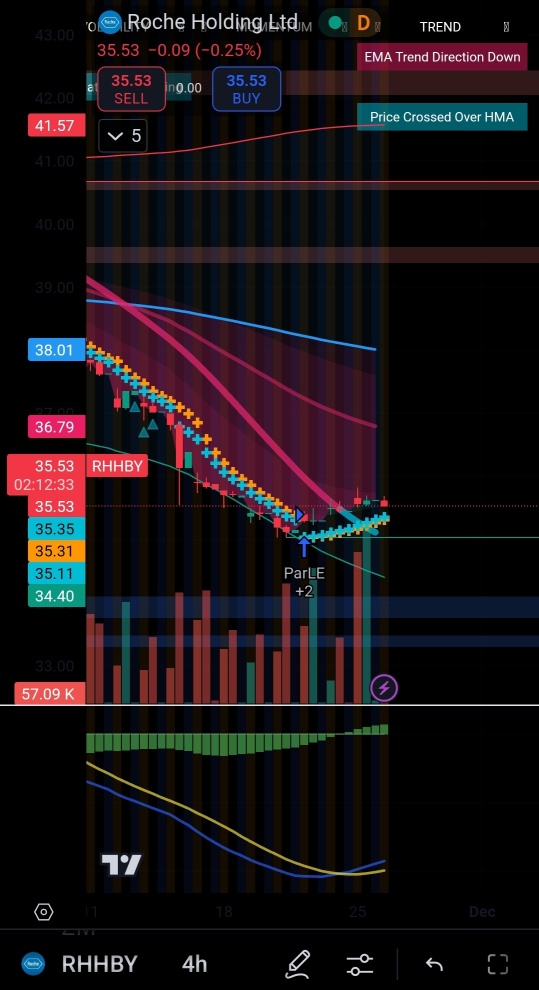
RHHBY ROCHE HOLDING AG
- 35.640
- -0.830-2.28%
15min DelayClose Dec 10 16:00 ET
- 35.640
- 0.0000.00%
Post 16:42 ET
227.18BMarket Cap18.93P/E (TTM)
36.370High35.610Low1.23MVolume36.350Open36.470Pre Close44.10MTurnover0.02%Turnover Ratio17.51P/E (Static)6.37BShares42.43052wk High6.90P/B227.18BFloat Cap29.20052wk Low1.36Dividend TTM6.37BShs Float49.895Historical High3.80%Div YieldTTM2.08%Amplitude-3.794Historical Low35.877Avg Price1Lot Size
Post-Market
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Reported Earlier, Roche's Genentech Presents Long-Term Data at ASH 2024 Highlighting Durable Remissions With Fixed-Duration Columvi And Lunsumio
Biogen Downgraded to Hold by Jefferies, Ocrevus Royalties Cited
Reported Sunday, Roche's Genentech Showcases Polivy's Transformative Impact On Frontline DLBCL Treatment: Five-Year POLARIX Trial Results Presented At ASH 2024
Kepler Capital Sticks to Their Buy Rating for Roche Holding AG (RHHVF)
FDA Accepts Roche's Columvi SBLA for Expanded Use in Lymphoma
Roche (RHHBY.US) CD20/CD3 bispecific antibody new indication application accepted by the FDA.
Roche (RHHBY.US) announced that the FDA has accepted the supplemental biological product license application (sBLA) for glofitamab.
Comments
$LumiraDx (LMDXF.US)$ currently under negotiations according to both SOFI & SHWABB. We have 1.2B in assets $Poseida Therapeutics (PSTX.US)$ had 250m in assets & 200m in liabilities got a banger of a deal at 1.5B evaluation from $ROCHE HOLDING AG (RHHBY.US)$ so even without overhang we are worth a 1:1 rhhby conversion based on how much of the company Roche got in the deal. That's not including if RonZ has plans for us with ElectraDX or if DNEDX has plans. 👀👀👀 could realistically be looking at 50.00...
9
$ROCHE HOLDING AG (RHHBY.US)$ High Revenue Medical ADR stock!
Meanwhile waiting for US stock like $Tesla (TSLA.US)$ $NVIDIA (NVDA.US)$ correction point, I am testing this ADR stock.
Meanwhile waiting for US stock like $Tesla (TSLA.US)$ $NVIDIA (NVDA.US)$ correction point, I am testing this ADR stock.
3
2
$LumiraDx (LMDXF.US)$ Lumiradx changing name to LKM INNOVATIONS and restructuring their finances? 🤔🤔🤔 ultimately Roche $ROCHE HOLDING AG (RHHBY.US)$ is in control but they still owe us 1.1B worse case scenario. I think this is what the dealio was with the shares transferring over. Let's hope Roche is on our side. I don't think they'd issue out their own directors and put this much effort in just to screw us holding shares. @BadPaint
4
3
$LumiraDx (LMDXF.US)$ I think we definitely have a leg to stand on IF we decided to proceed with legal action. The mere fact that shares would transfer was in SEC filings is enough evidence to pursue legal action if need be. Also keeping us in the dark all this time is illegal. If Roche $ROCHE HOLDING AG (RHHBY.US)$ does not deliver something soon I will be hiring a lawyer for sure. I hope it doesn't come to that and Roche rectifies this obnoxious behavior.
I still have plenty of optimism here th...
I still have plenty of optimism here th...
10
4
Read more
Heat List
Overall
Symbol
Price
% Chg
No Data