No Data
US Stock MarketDetailed Quotes
SNY Sanofi
- 48.150
- -0.040-0.08%
Close Jan 3 16:00 ET
- 48.150
- 0.0000.00%
Post 20:01 ET
120.72BMarket Cap26.13P/E (TTM)
48.160High47.790Low1.56MVolume48.090Open48.190Pre Close74.82MTurnover0.07%Turnover Ratio21.82P/E (Static)2.51BShares58.97052wk High1.62P/B106.20BFloat Cap43.38152wk Low2.10Dividend TTM2.21BShs Float58.970Historical High4.35%Div YieldTTM0.77%Amplitude8.844Historical Low48.007Avg Price1Lot Size
Full Hours
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Sanofi (SNY.US) has received approval for clinical trials of its novel dual antibody drug in China.
Recently, CDE announced that the Class 1 new drug SAR442970 injection declared by Sanofi (SNY.US) has obtained implied approval for clinical trials.
Brand Prescription Drugs to Rise an Average 4.5% in 2025
Market Chatter: Drug Companies to Raise Prices on 250 Branded Drugs in 2025
Sanofi (SNY.US) has applied for the market launch of BTK inhibitors in China.
On December 30, the CDE official website showed that Sanofi (SNY.US) has had its market application for the BTK inhibitor Rilzabrutinib accepted.
How Is The Market Feeling About Sanofi?
RTW Biotech Opportunities Unit Closes Rights Deal With Sanofi, Buys Investigational Drug
Comments
The market fell Tuesday, but stepping back, the view of tech stocks looks like a mountain range that has not peaked yet- for all of December, the Nasdaq has arched upward into all-time highs. After Monday's record, the index pulled back, following a pullback for Nvidia and Broadcom, falling after Broadcom's post-earnings stock jump finally took a pause.
Just past 4 pm ET they $S&P 500 Index (.SPX.US)$ traded down 0.39%,...
Just past 4 pm ET they $S&P 500 Index (.SPX.US)$ traded down 0.39%,...

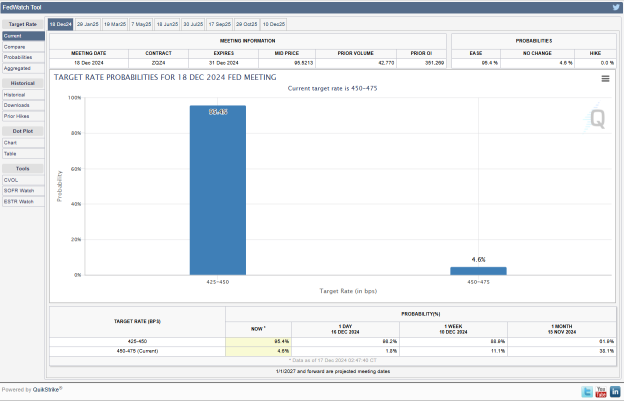
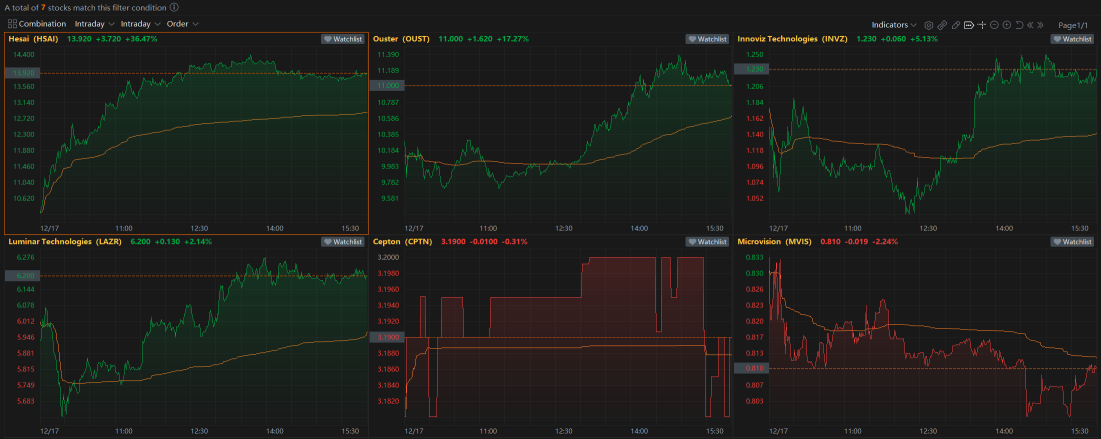
16
3
$Esperion Therapeutics (ESPR.US)$ Acquiring Esperion Therapeutics could be a strategic move for a big pharmaceutical company for several compelling reasons. Here are the main factors that suggest an acquisition could be beneficial for a larger player in the pharmaceutical industry:
1. Unique Market Position in Statin Alternatives
– Addressing Statin Intolerance: There are millions of statin...
1. Unique Market Position in Statin Alternatives
– Addressing Statin Intolerance: There are millions of statin...
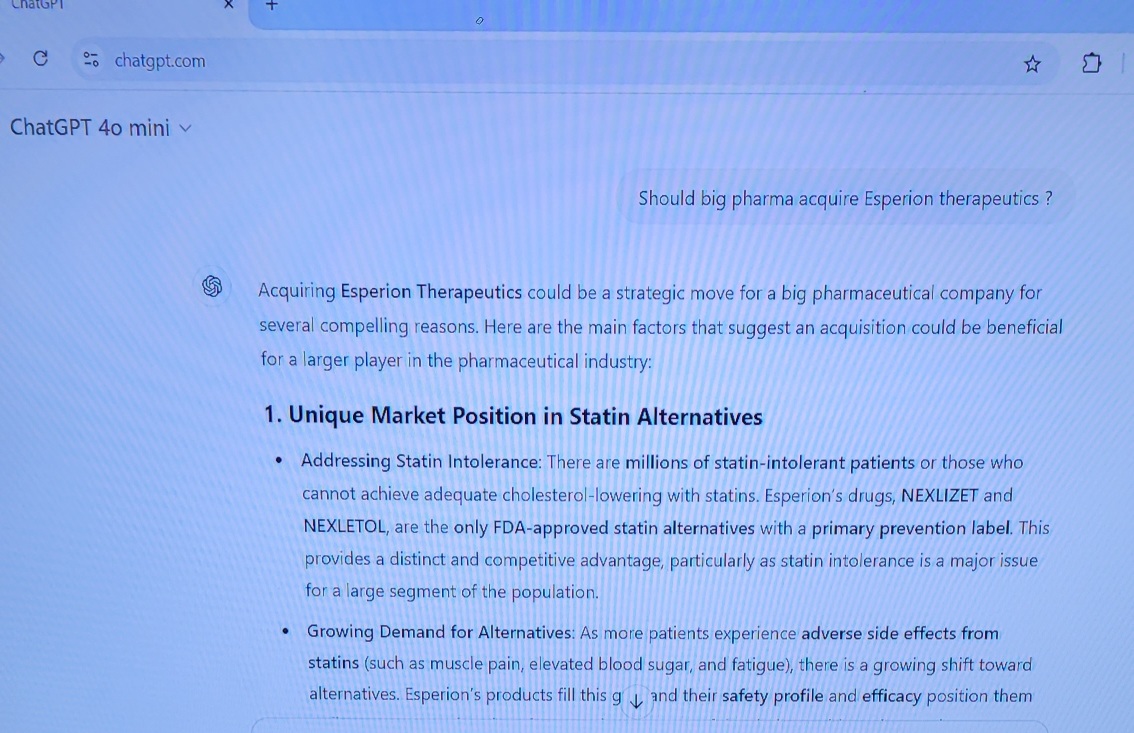
10
2
4
🚨 This Week’s PDUFAs:
$Heron Therapeutics (HRTX.US)$ : 🤔
⇨ ZYNRELEF® Vial Access Needle
‣ Postoperative pain
‣ PDUFA: 9/23/24 (sNDA)
$Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ KarXT (xanomeline-trospium)
‣ Schizophrenia
‣ PDUFA: 9/26/24 (NDA)
$Sanofi (SNY.US)$ $Regeneron Pharmaceuticals (REGN.US)$ : 🤔
⇨ Dupixent
‣ Chronic obstructive pulmonary disease
‣ PDUFA: 9/27/24 (sBLA)
$BeiGene (BGNE.US)$ $Merck & Co (MRK.US)$ & $Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ Tislelizumab, KEYTRUDA, OPDIVO & YER...
$Heron Therapeutics (HRTX.US)$ : 🤔
⇨ ZYNRELEF® Vial Access Needle
‣ Postoperative pain
‣ PDUFA: 9/23/24 (sNDA)
$Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ KarXT (xanomeline-trospium)
‣ Schizophrenia
‣ PDUFA: 9/26/24 (NDA)
$Sanofi (SNY.US)$ $Regeneron Pharmaceuticals (REGN.US)$ : 🤔
⇨ Dupixent
‣ Chronic obstructive pulmonary disease
‣ PDUFA: 9/27/24 (sBLA)
$BeiGene (BGNE.US)$ $Merck & Co (MRK.US)$ & $Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ Tislelizumab, KEYTRUDA, OPDIVO & YER...
9
1
📣 ITRM AdCom 9/9 meeting ongoing 🚗
👀 stay tuned for updates on meeting outcomes
See 👇 for further info.
🚨 This Week’s AdCom:
$Iterum Therapeutics (ITRM.US)$ : Meeting ongoing 🤔
⇨ Sulopenem
‣ uUTI (Uncomplicated Urinary Tract Infection)
‣ AdCom: 9/9/24
🚨 Last Week’s PDUFAs:
$Travere Therapeutic (TVTX.US)$ : Approved 9/5 🎉
⇨ FILSPARI (Sparsentan)
‣ IgA nephropathy (IgAN)
‣ PDUFA: 9/5/24
$Avadel Pharmaceuticals (AVDL.US)$ : Awaiting decision 🤔
⇨ LUMRYZ (sodium oxybate)
‣...
👀 stay tuned for updates on meeting outcomes
See 👇 for further info.
🚨 This Week’s AdCom:
$Iterum Therapeutics (ITRM.US)$ : Meeting ongoing 🤔
⇨ Sulopenem
‣ uUTI (Uncomplicated Urinary Tract Infection)
‣ AdCom: 9/9/24
🚨 Last Week’s PDUFAs:
$Travere Therapeutic (TVTX.US)$ : Approved 9/5 🎉
⇨ FILSPARI (Sparsentan)
‣ IgA nephropathy (IgAN)
‣ PDUFA: 9/5/24
$Avadel Pharmaceuticals (AVDL.US)$ : Awaiting decision 🤔
⇨ LUMRYZ (sodium oxybate)
‣...
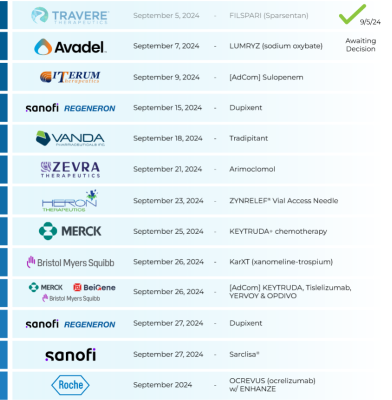
5
1
$Vir Biotechnology (VIR.US)$ $Sanofi (SNY.US)$ Reuters· 4 mins ago
Vir Biotechnology Announces Closing of Exclusive Worldwide License Agreement With Sanofi for Multiple Potential Best-in-Class Clinical-Stage T-Cell Engagers
Vir Biotechnology Announces Closing of Exclusive Worldwide License Agreement With Sanofi for Multiple Potential Best-in-Class Clinical-Stage T-Cell Engagers
1
Read more
Trending US Stocks

U.S Tech Companies U.S Tech Companies
U.S leading technology companies with strong market presence, influential in their industries, and notable for robust innovation and profitability. Information is provided by Futu and is a non-exhaustive list of all thematic stocks for reference purposes only.
This section presents the top 5 stocks in U.S Tech Companies, ranked from highest to lowest based on real-time market data. U.S leading technology companies with strong market presence, influential in their industries, and notable for robust innovation and profitability. Information is provided by Futu and is a non-exhaustive list of all thematic stocks for reference purposes only.
This section presents the top 5 stocks in U.S Tech Companies, ranked from highest to lowest based on real-time market data.


