No Data
SNY241115C65000
- 0.29
- 0.000.00%
- 5D
- Daily
News
Sanofi's new drug Lunsekimig injection was approved for clinical trials in China again, intended for the treatment of asthma.
The official website of the China National Medical Products Administration (NMPA) recently announced that the class 1 new drug lunsekimig injection submitted by Sanofi (SNY.US) has obtained a new implied license for clinical trials, intended to be developed for the treatment of high-risk asthma in adults.
Sanofi Treatment of Acute Myeloid Leukemia Granted Orphan Status
MediciNova Shares Are Trading Higher After the Company Announced That It Was Notified by Sanofi That the Sanofi/Novartis Litigation Was Settled.
Novavax Falls After Guidance Cut on Lower Vaccine Sales
Zucara Therapeutics Announces Strategic Investment From Sanofi as Part of US$20 Million Series B Financing
Express News | Medicinova Given Notice of Monetary Damages Due Under Patent Settlement of Sanofi-Novartis
Comments
$Heron Therapeutics (HRTX.US)$ : 🤔
⇨ ZYNRELEF® Vial Access Needle
‣ Postoperative pain
‣ PDUFA: 9/23/24 (sNDA)
$Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ KarXT (xanomeline-trospium)
‣ Schizophrenia
‣ PDUFA: 9/26/24 (NDA)
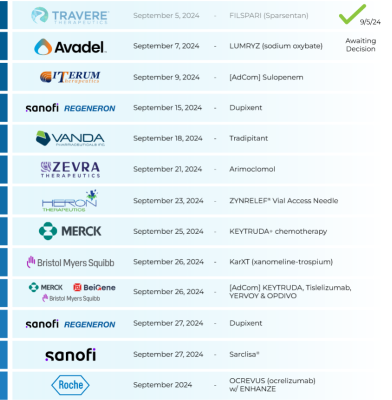
$Sanofi (SNY.US)$ $Regeneron Pharmaceuticals (REGN.US)$ : 🤔
⇨ Dupixent
‣ Chronic obstructive pulmonary disease
‣ PDUFA: 9/27/24 (sBLA)
$BeiGene (BGNE.US)$ $Merck & Co (MRK.US)$ & $Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ Tislelizumab, KEYTRUDA, OPDIVO & YER...
👀 stay tuned for updates on meeting outcomes
See 👇 for further info.
🚨 This Week’s AdCom:
$Iterum Therapeutics (ITRM.US)$ : Meeting ongoing 🤔
⇨ Sulopenem
‣ uUTI (Uncomplicated Urinary Tract Infection)
‣ AdCom: 9/9/24
🚨 Last Week’s PDUFAs:
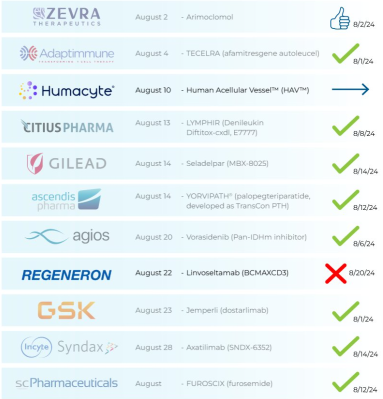
$Travere Therapeutic (TVTX.US)$ : Approved 9/5 🎉
⇨ FILSPARI (Sparsentan)
‣ IgA nephropathy (IgAN)
‣ PDUFA: 9/5/24
$Avadel Pharmaceuticals (AVDL.US)$ : Awaiting decision 🤔
⇨ LUMRYZ (sodium oxybate)
‣...
Vir Biotechnology Announces Closing of Exclusive Worldwide License Agreement With Sanofi for Multiple Potential Best-in-Class Clinical-Stage T-Cell Engagers
The stock recently closed at $113.65, with a consensus price target of $117.74, indicating a potential upside of 3.60%.
Sanofi's market cap stands at $141.1 billion, with a P/E ratio of 30.47.
$Sanofi (SNY.US)$
$Travere Therapeutic (TVTX.US)$ : FILSPARI (Sparsentan)
$Avadel Pharmaceuticals (AVDL.US)$ : LUMRYZ (FT218)
$Iterum Therapeutics (ITRM.US)$ : Sulopenem - AdCom 🗓️
$Sanofi (SNY.US)$ & $Regeneron Pharmaceuticals (REGN.US)$ : Dupixent
$Vanda Pharmaceuticals (VNDA.US)$ : Tradipitant
$Zevra Therapeutics (ZVRA.US)$ : Arimoclomol
$Heron Therapeutics (HRTX.US)$ : ZYNRELEF® Vial Access Needle
$Merck & Co (MRK.US)$ : KEYTRUDA+ chemotherapy
$Bristol-Myers Squibb (BMY.US)$ : KarXT (...