No Data
US Stock MarketDetailed Quotes
VIR Vir Biotechnology
- 7.340
- 0.0000.00%
Close Dec 23 16:00 ET
- 7.340
- 0.0000.00%
Post 16:01 ET
1.01BMarket Cap-1.87P/E (TTM)
7.520High7.260Low770.97KVolume7.390Open7.340Pre Close5.69MTurnover0.83%Turnover RatioLossP/E (Static)137.72MShares13.09052wk High0.81P/B679.07MFloat Cap6.56052wk Low--Dividend TTM92.52MShs Float141.010Historical High--Div YieldTTM3.54%Amplitude6.560Historical Low7.375Avg Price1Lot Size
Post-Market
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Express News | US FDA: Revoked Emergency Use Authorization for GSK and Vir Biotechnology's Sotrovimab as of Dec 13 - Website
Vir Biotechnology to Present at the 43rd Annual J.P. Morgan Healthcare Conference
Vir Gets FDA Breakthrough Therapy Status for Two Drugs
Express News | Vir Biotechnology Inc - Phase 3 Eclipse Program to Begin in First Half of 2025
Express News | Vir Biotechnology Receives FDA Breakthrough Therapy Designation and EMA Prime Designation for Tobevibart and Elebsiran in Chronic Hepatitis Delta
Vir Biotechnology Receives FDA Breakthrough Therapy Designation and EMA PRIME Designation for Tobevibart and Elebsiran in Chronic Hepatitis Delta
Comments
$Vir Biotechnology (VIR.US)$ $Eli Lilly and Co (LLY.US)$ Reuters· 2 mins ago
US FDA: Revoked Emergency Use Authorization for GSK and Vir Biotechnology's Sotrovimab as of Dec 13 - Website
US FDA: Revoked Emergency Use Authorization for GSK and Vir Biotechnology's Sotrovimab as of Dec 13 - Website
NOTE: Whenever a stock is up, make sure you check its dilution risk - cash on hand because most likely if they are cash-strapped, they will make offering. Scaling out is key. NEVER listen to those who say hold on because if you lose money, they wouldnt care AT ALL.
*************************************
REVERSE...
*************************************
REVERSE...
60
8
$Vir Biotechnology (VIR.US)$ Reuters· 16:05
Vir Biotechnology Receives FDA Breakthrough Therapy Designation and EMA Prime Designation for Tobevibart and Elebsiran in Chronic Hepatitis Delta
Vir Biotechnology Receives FDA Breakthrough Therapy Designation and EMA Prime Designation for Tobevibart and Elebsiran in Chronic Hepatitis Delta
2
$Nauticus Robotics (KITT.US)$ $Kindly MD (KDLY.US)$ $Jayud Global Logistics Ltd. (JYD.US)$ $Mobilicom (MOB.US)$ $Vir Biotechnology (VIR.US)$ $Arqit Quantum (ARQQ.US)$ $Serina Therapeutics (SER.US)$ $Credo Technology (CRDO.US)$ $Citius Oncology (CTOR.US)$ $Shimmick (SHIM.US)$ $Janux Therapeutics (JANX.US)$ $Volato Group (SOAR.US)$ $Cheetah Net Supply Chain Service (CTNT.US)$
📊⚡️📊
📊⚡️📊
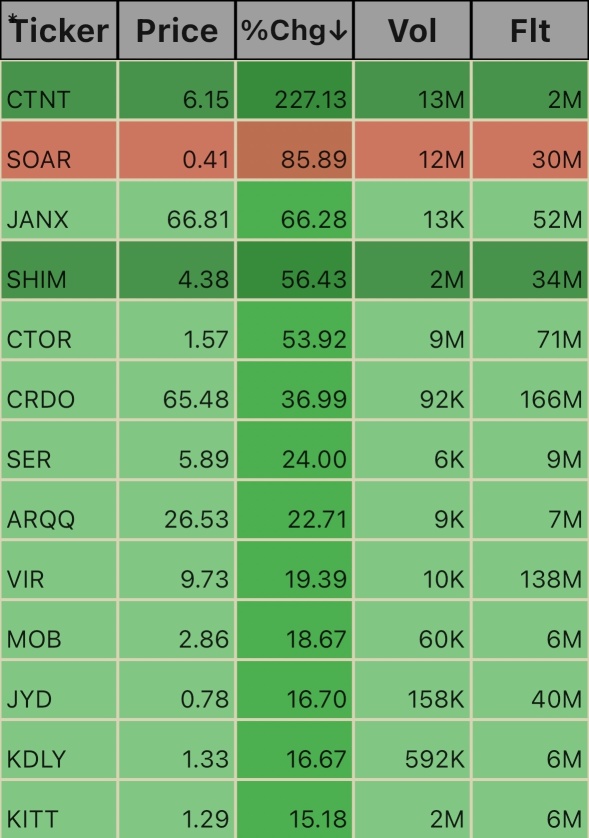
Note: This seems to be a long list. The rationale is that I al focused on at least 20% gains and not just after bangers. My trading style is to compound those 10-15-20-30% earnings and roll all profit rather than waiting for runners everyday. If runners appear, then I’ll grab it as well. Each has his own style earning.
$Damon Inc (DMN.US)$ Can be my PM gapper. Low float EV stock. IPO las...
$Damon Inc (DMN.US)$ Can be my PM gapper. Low float EV stock. IPO las...
35
19
5
Read more
Heat List
Overall
Symbol
Price
% Chg
No Data

 my friend
my friend

