 August P/L Challenge: Have you been active in trading this month?
August P/L Challenge: Have you been active in trading this month?
Views 1.9M
Contents 110

Patience you must have, my young Padawan.
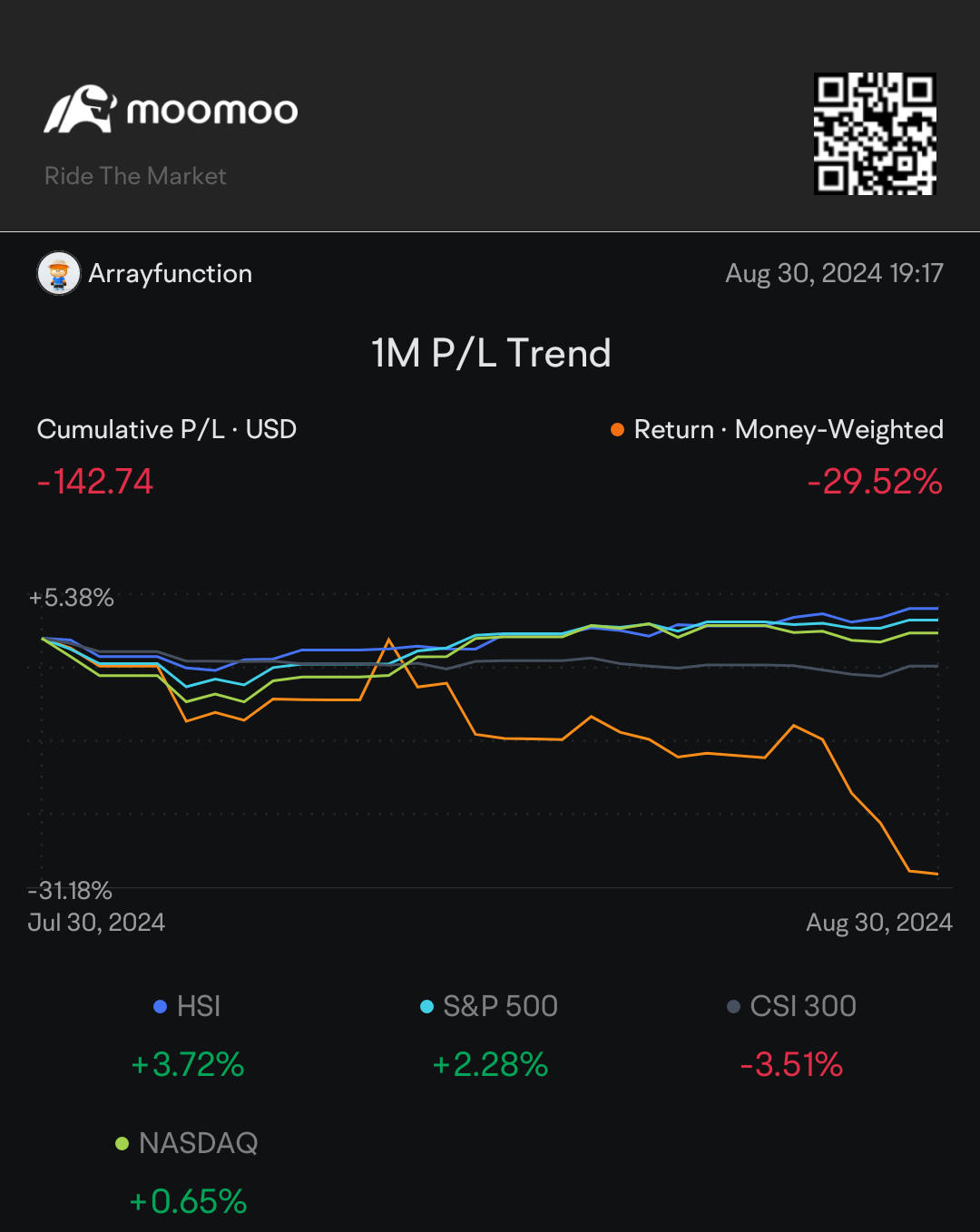
August was an interesting month for sure! Health concerns led me to close out a lot of equities for more liquidity (primarily 5% APay no penalty CDs). I couldn't figure out how to get Moomoo to not count withdrawal as losses, sorry in advance!
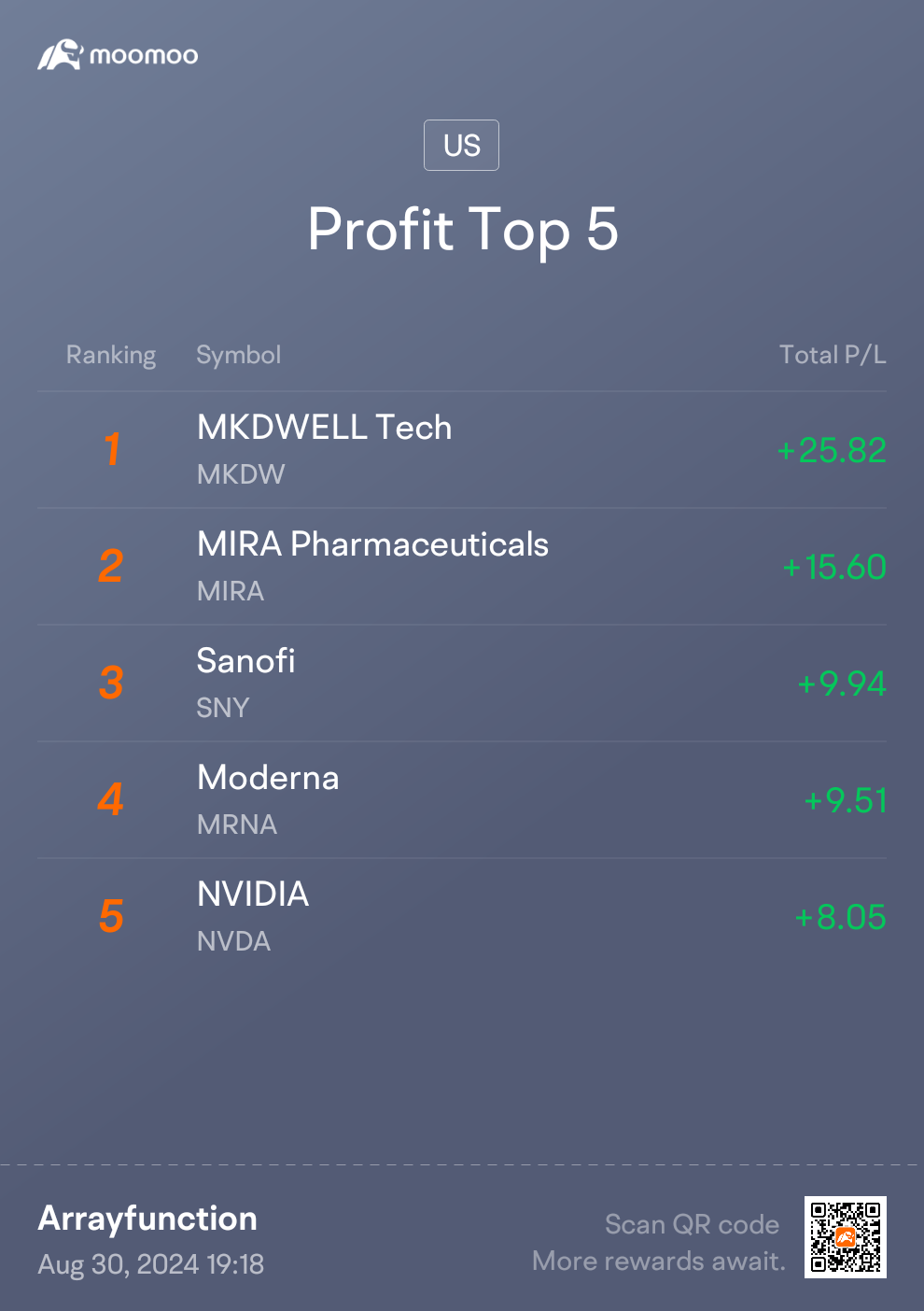
I think the month went fairly well overall! Always new lessons to learn through 🙃 It took all of a few days after making solid returns on a SPAC to lose more on a different SPAC. I am now done playing with those 😂
I still don't quite understand why Nvidia took such a drop after beating estimates. One of life's fun little jokes; the one time I set concerns over an AI bubble and aversion to leveraged ETFs is when good news tanks a price 🫠
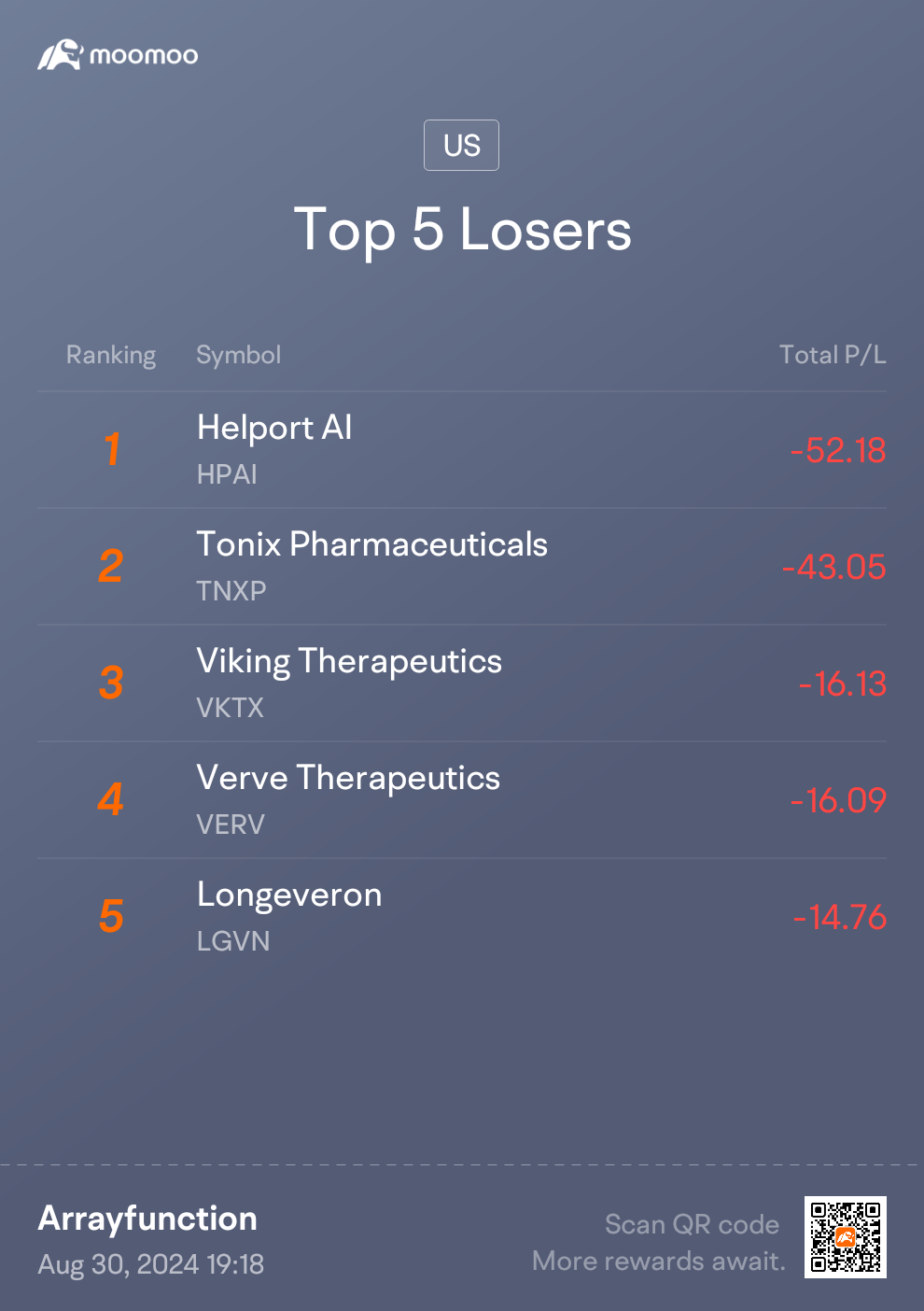
Biggest lesson was the power of patience and fighting the lizard brain's aversion to red! The only equity I didn't close out on Moomoo was
$Tonix Pharmaceuticals (TNXP.US)$. My reason for investing in TNXP has always been the upcoming NDA on the fibromyalgia medication.
Getting caught up in the MPOX hype was just an added bonus that let me make a fair bit on just swinging the stock on the high/low cycles. I simply kept reinvesting those returns into more shares. The hype dying down has also proved fortunate for me! I think of it as the shares being on sale (and the discount keeps growing) and have continued to load up.
I have always struggled with the discipline to ignore those red numbers getting higher and higher, but having an actual written plan made it a lot easier! I decided before buying that I would hold until FDA approval. Just that level of a plan now let's me ignore those red numbers. I highly recommend trying it, especially new traders like me, if you struggle with being quick to cut your losses!
I also transitioned over from the margin account to the cash account this month. For whatever reason, Moomoo can't process transfers-in-kind like other brokers. But it was good timing! I happened to be closing out all my positions in the margin account at the same time I decided to move into more liquid investments.
And of course - the important disclaimer - don't go speculating in biotechs with money you can't 100% afford to lose! TNXP still could very much go up in flames 😂
If you want to dabble in the arena, be sure to learn how the drug pipelines and businesses operate! I also would recommend taking a fundamental analysis approach over technical. You can greatly reduce your risk if you stick with companies that have enough of a war chest to manage debt payments and pay for operations. Having multiple product pipelines can also help minimize risk and offer buy-in opportunities. Say a company's stock tanks after bad phase 2 data but they are a phase 3 trial nearly wrapped up. Wait for the panic selling for the bad news to happen, then pick up the shares and wait for that phase 3 to be approved.
I try to leave random posts on my page outlining certain parts of the biotech business operations (not related to trading, just in general) so always happy to help folks grasp how weird the sector is!
Disclaimer: Community is offered by Moomoo Technologies Inc. and is for educational purposes only.
Read more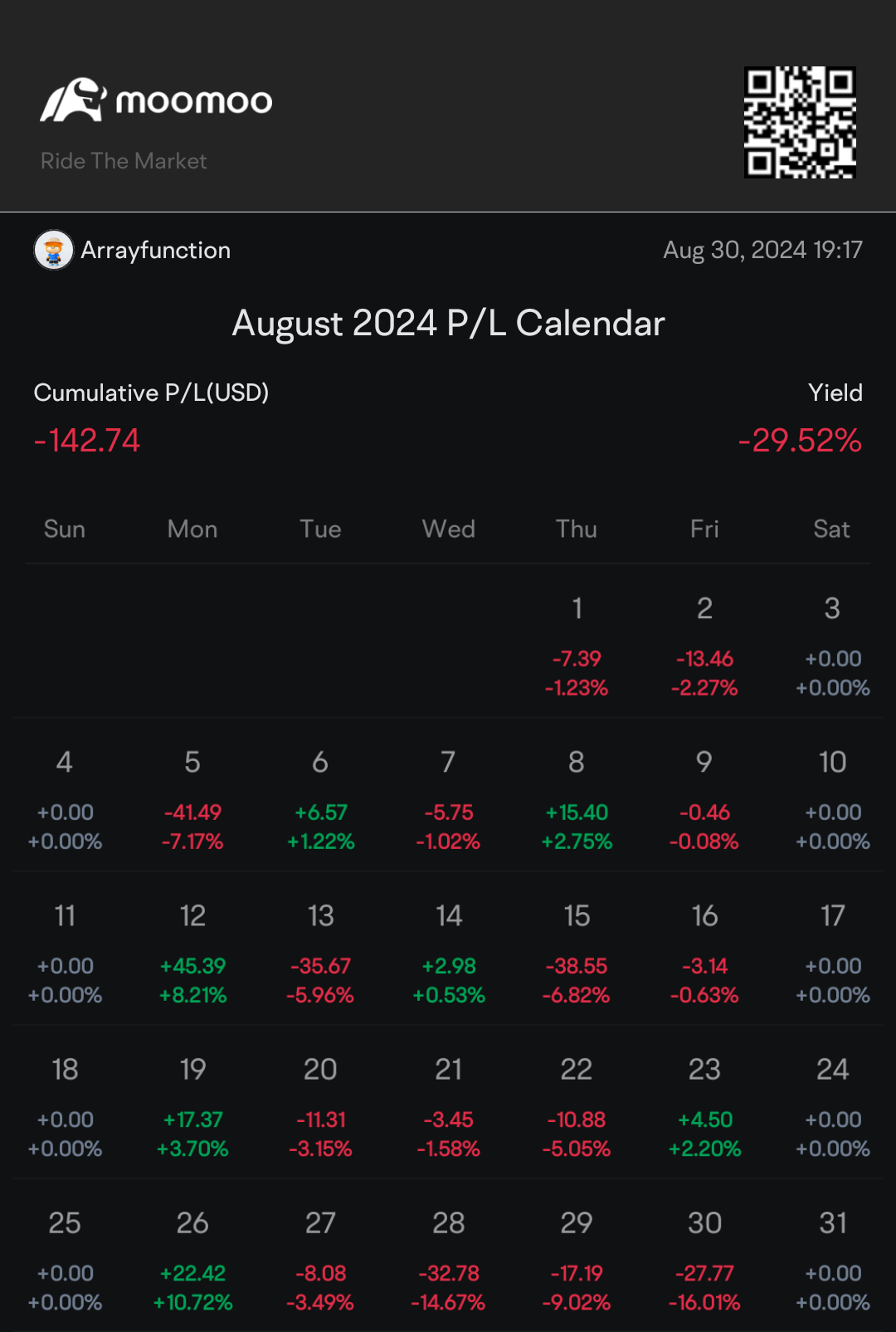
LIMCHIKEEN : I like your view. because of the red, I missed out on Phathom pharma.
XJJX : TNXP can be said to be the worst, and it will continue to fall on Monday.
Arrayfunction OP XJJX : Yep, you are right! It almost certainly will fall! They are in desperate need of cash if they want to keep all their plates spinning in the air. Hence tripling the stock offering.
It would be a horrible stock to swing trade on, as you say. I am only confident in holding because I am very well versed in the science and markets of fibromyalgia and chronic pain treatments. So I can read their phase 3 results and know the FDA has no reason to stall that NDA beyond the end of the year.
If it were a completely novel molecule or something like a cancer treatment I don't understand the market potential of very well, then I definitely wouldn't be staying in
Arrayfunction OP LIMCHIKEEN : Are you talking about Voquenza's approval? I guess it says something about me that I have GERD and never heard of the drug until just now
But you do make a great point that going long on biotechs is a case-by-case basis! A drug getting approved doesn't mean it is guaranteed to gain market share at all.
There are a few rules of thumb I use from looking at the balance sheet - but only to the extent of red flags to rule them out. Since biotechs generate no revenue for years, debt is far more dangerous.
For an average company, (e.g. no plans for an NDA within 6-8 months) I don't hold if they have debt > 15% of assets. Once they start down the debt financing spiral, it's game over man (barring a deus ex machina like a huge grant or angel investor). They can usually limp along for a while by borrowing at increasing interest to make past debt payments. But as the Euro crisis showed, it only works for so long. They can cut costs by abandoning earlier stage work - but often not while a trial is actively ongoing due to contractual obligations.
It's also very important to be comfortable admitting what you don't know! There are sectors in drug development I have a lot of knowledge in from personal research (e.g. depression, anxiety, chronic pain, auto-immune, neurological disorders) but plenty that I don't. If I can't get at least a rough feel for what the market potential and what the demand will be, I wouldn't go long.
That's largely why I avoid cancer drugs. I simply don't know enough about the market to know how easy / hard it will be to capture market share. For the US market, you always need to factor in insurance companies also. They obviously are going to fight tooth and nail not to pay for any new name brand drug. If it's entering the market as being simply better than existing generics, insurance companies will insist people try the cheaper ones first.
Arrayfunction OP Arrayfunction OP : For example, for me to get coverage for a newer anti-depressant therapy (with ~70% efficacy) my insurance company required that I submit proof I "failed" on at least 4 other medications. In that case, I did have those records already. Otherwise, since the "failure" timeframe is 6-8 weeks minimum on anti-depressants, the treatment would have been on the market almost a year before I could access it.

If something gets approved by Medicare, that's about as close as possible to a guaranteed adoption won't be stalled.
Which is why Voquenza is so curious! GERD can be managed extremely well with generic proton pump inhibitors (e.g. Omeprazole). There is a growing body of evidence to show chronic long-term PPI usage can damage bone health (since it limits calcium absorption). But insurance companies will insist as long as legally possible that such a risk is not enough reason to pay for a brand name drug
Jrithe : I like your view. Good job! bro
Stella-K : good luck for Q4!
Rollingling : you are so coooool!