No Data
US Stock MarketDetailed Quotes
MDCX Medicus Pharma
- 2.800
- +0.020+0.72%
Close Dec 11 16:00 ET
- 3.110
- +0.310+11.07%
Pre 08:02 ET
33.09MMarket Cap-1.35P/E (TTM)
3.000High2.790Low8.36KVolume2.790Open2.780Pre Close23.85KTurnover0.20%Turnover RatioLossP/E (Static)11.82MShares5.00052wk High5.13P/B11.76MFloat Cap1.80052wk Low--Dividend TTM4.20MShs Float5.000Historical High--Div YieldTTM7.55%Amplitude1.800Historical Low2.853Avg Price1Lot Size
Pre-Market
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Express News | Medicus Pharma Says INAD File No.013880 Received Minor Use In Major Species Designation From FDA For Its Dissolvable D-MNA To Treat External Squamous Cell Carcinoma In Horses
Medicus Pharma Announcs Its INAD Received MUMS From U.S. FDA
Medicus Pharma Ltd. Announces Minor Use (MUMS) Designation From the FDA for Doxorubicin-Containing Microneedle Array (D-MNA) Patch
Medicus Pharma Eyes Pivotal Trial for Novel Skin Cancer Therapy as Phase 2 Enrollment Progresses | TSX-V:MDCX, NASDAQ:MDCX
Medicus Pharma Enters Collaboration Agreement With Swanielle
Medicus Pharma Ltd. Announces Collaboration Agreement to Expand Phase 2 Clinical Study in Asia Pacific Region
Comments
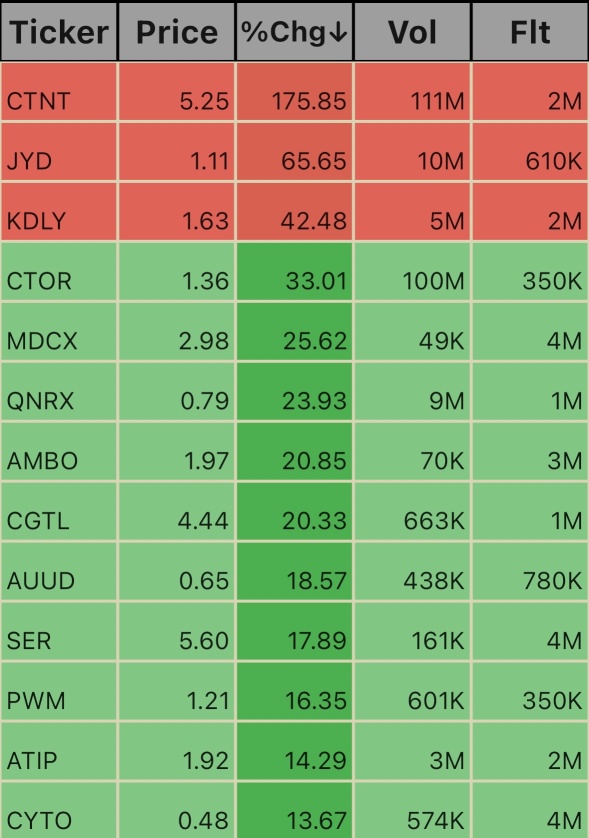
$Altamira Therapeutics (CYTO.US)$ $ATI Physical Therapy (ATIP.US)$ $Prestige Wealth (PWM.US)$ $Serina Therapeutics (SER.US)$ $Auddia (AUUD.US)$ $Creative Global Technology (CGTL.US)$ $Ambow Education (AMBO.US)$ $Quoin Pharmaceuticals (QNRX.US)$ $Citius Oncology (CTOR.US)$ $Medicus Pharma (MDCX.US)$ $Kindly MD (KDLY.US)$ $Jayud Global Logistics Ltd. (JYD.US)$ $Cheetah Net Supply Chain Service (CTNT.US)$
📊⚡️📊
📊⚡️📊
4
$Medicus Pharma (MDCX.US)$
Medicus Pharma Ltd. Announces Collaboration Agreement to Expand Phase 2 Clinical Study in Asia Pacific Region
Monday, 2nd December at 7:30 am
TORONTO, Dec. 02, 2024 (GLOBE NEWSWIRE) -- Medicus Pharma Ltd. (NASDAQ: MDCX) (TSXV: MDCX) ("Medicus" or the "Company") is pleased to announce an agreement with Swanielle Inc. ("Swanielle") to explore expansion of Phase 2 clinical study for treatment of Basal Cell Carcinoma (BCC) in the Asia-pacific region
.
Under the agreemen...
Medicus Pharma Ltd. Announces Collaboration Agreement to Expand Phase 2 Clinical Study in Asia Pacific Region
Monday, 2nd December at 7:30 am
TORONTO, Dec. 02, 2024 (GLOBE NEWSWIRE) -- Medicus Pharma Ltd. (NASDAQ: MDCX) (TSXV: MDCX) ("Medicus" or the "Company") is pleased to announce an agreement with Swanielle Inc. ("Swanielle") to explore expansion of Phase 2 clinical study for treatment of Basal Cell Carcinoma (BCC) in the Asia-pacific region
.
Under the agreemen...
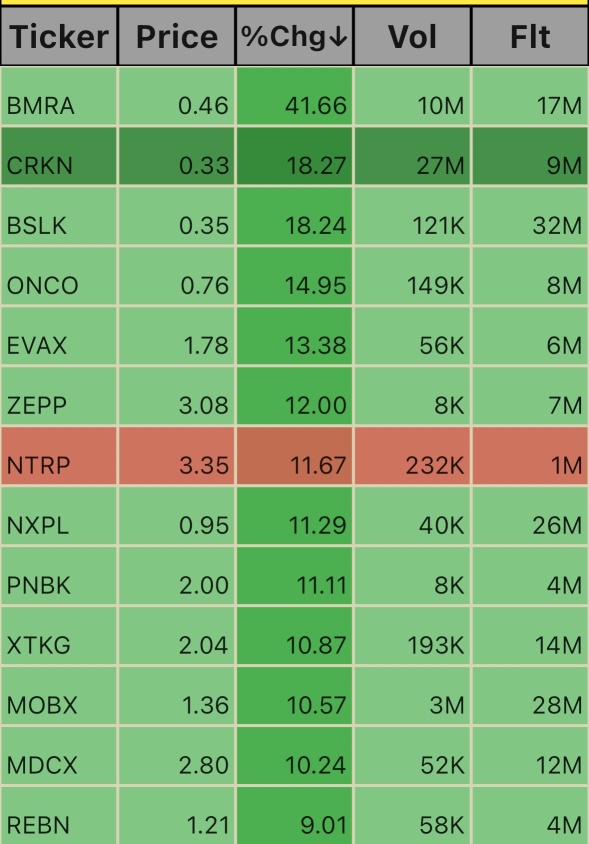
$Reborn Coffee (REBN.US)$ $Medicus Pharma (MDCX.US)$ $Mobix Labs (MOBX.US)$ $X3 Holdings (XTKG.US)$ $Pacific Mercantile Bancorp (PNBK.US)$ $NextPlat (NXPL.US)$ $NextTrip (NTRP.US)$ $Zepp Health (ZEPP.US)$ $Evaxion Biotech (EVAX.US)$ $Onconetix (ONCO.US)$ $Bolt Projects (BSLK.US)$ $Crown Electrokinetics (CRKN.US)$ $Biomerica (BMRA.US)$
📊⚡️📊
📊⚡️📊
4
5
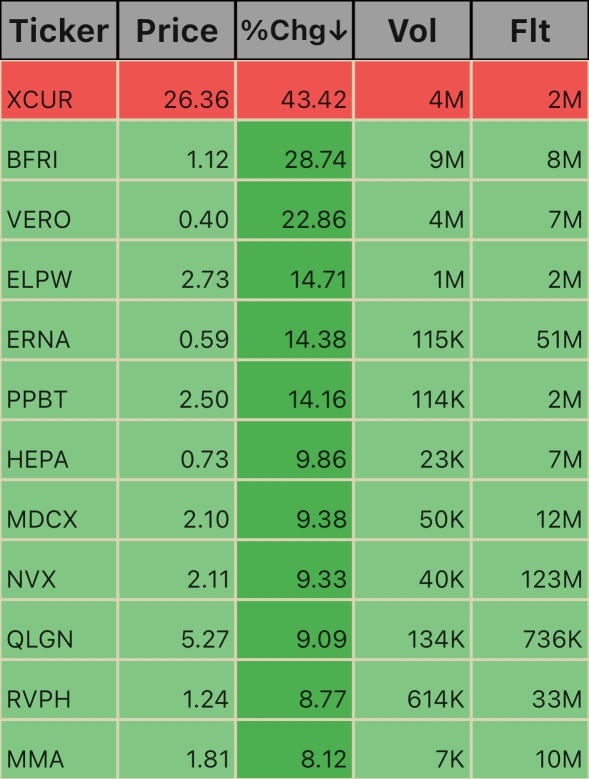
$Mixed Martial Arts Group (MMA.US)$ $Reviva Pharmaceuticals (RVPH.US)$ $Qualigen Therapeutics (QLGN.US)$ $NOVONIX (NVX.US)$ $Medicus Pharma (MDCX.US)$ $Hepion Pharmaceuticals (HEPA.US)$ $Purple Biotech (PPBT.US)$ $Eterna Therapeutics (ERNA.US)$ $Elong Power (ELPW.US)$ $Venus Concept (VERO.US)$ $Biofrontera (BFRI.US)$ $Exicure (XCUR.US)$
📊⚡️📊
📊⚡️📊
2
1
$Medicus Pharma (MDCX.US)$
Medicus Pharma Ltd. Announces Closing of US$4.0M Initial Public Offering in the United States
Medicus Pharma Ltd. Announces Closing of US$4.0M Initial Public Offering in the United States
Read more
Trending US Stocks
U.S. Crypto Concept Stocks U.S. Crypto Concept Stocks
Companies involved in the creation, trade, and services of digital forms of money.Displayed third-party logos, brands, or trademark images on screens or web pages are only for identification purposes and remain the property of their respective owners.Displayed third-party logos, brands, or trademark images on screens or web pages are only for identification purposes and remain the property of their respective owners. Information is provided by Futu and is a non-exhaustive list of all thematic stocks for reference purposes only.
This section presents the top 5 stocks in U.S. Crypto Concept Stocks, ranked from highest to lowest based on real-time market data. Companies involved in the creation, trade, and services of digital forms of money.Displayed third-party logos, brands, or trademark images on screens or web pages are only for identification purposes and remain the property of their respective owners.Displayed third-party logos, brands, or trademark images on screens or web pages are only for identification purposes and remain the property of their respective owners. Information is provided by Futu and is a non-exhaustive list of all thematic stocks for reference purposes only.
This section presents the top 5 stocks in U.S. Crypto Concept Stocks, ranked from highest to lowest based on real-time market data.