美股期權個股詳情
MESO241220P18000
- 0.00
- 0.000.00%
延時15分鐘行情收盤價 11/26 09:30 (美東)
0.00最高價0.00最低價
0.00今開0.00昨收0張成交量0張未平倉合約數18.00行權價0.00成交額169.87%引伸波幅-62.90%溢價2024/12/20到期日6.95内在价值100合約乘數23天距到期日0.00時間價值100合約規模美式期權類型-0.8200Delta0.0561Gamma1.51槓桿倍數-0.0250Theta-0.0080Rho-1.24有效槓桿0.0074Vega
Mesoblast股票討論
$Mesoblast (MESO.US)$ …got approval from FDA??
1
$Mesoblast (MESO.US)$ How low will it go?
1
1
$Mesoblast (MESO.US)$ … today back to 10
1
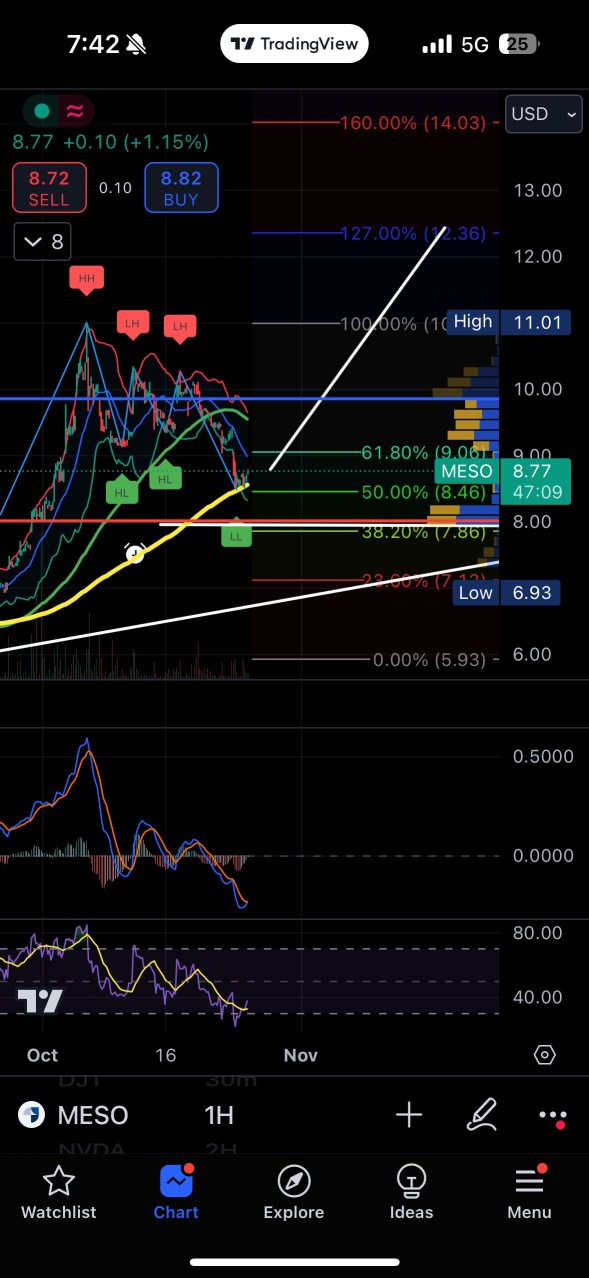
7
$Mesoblast (MESO.US)$ .. will drop then rocket by end of this year
1
2
$Mesoblast (MESO.US)$
Mesoblast Option to Issue Up to US$50 Million Convertible Notes for Product Launch | MESO Stock News
Mesoblast Option to Issue Up to US$50 Million Convertible Notes for Product Launch | MESO Stock News

1
1
$Mesoblast (MESO.US)$ .. if below to 5.. nice to buy!!!

2
4
$Mesoblast (MESO.US)$ Mesoblast (MESO) has released an update. Mesoblast Limited has resubmitted a Biologics License Application (BLA) to the FDA for Ryoncil®, aiming to treat children with Steroid-Refractory Acute Graft-Versus-Host Disease (SR-aGVHD). The resubmission, buoyed by promising Phase 3 study results demonstrating significant survival benefits, could meet an urgent need for effective treatment in this patient group, as current survival outcomes are poor and no FDA-approved treatm...
2
8
$Mesoblast (MESO.US)$ which is developing off-the-shelf cellular medicines for life-threatening inflammatory conditions, is planning to file a Biologics License Application for Ryoncil with the FDA next week.
2
2
暫無評論




Aariya : 可能快到達或已經完成,但尚未發表。