No Data
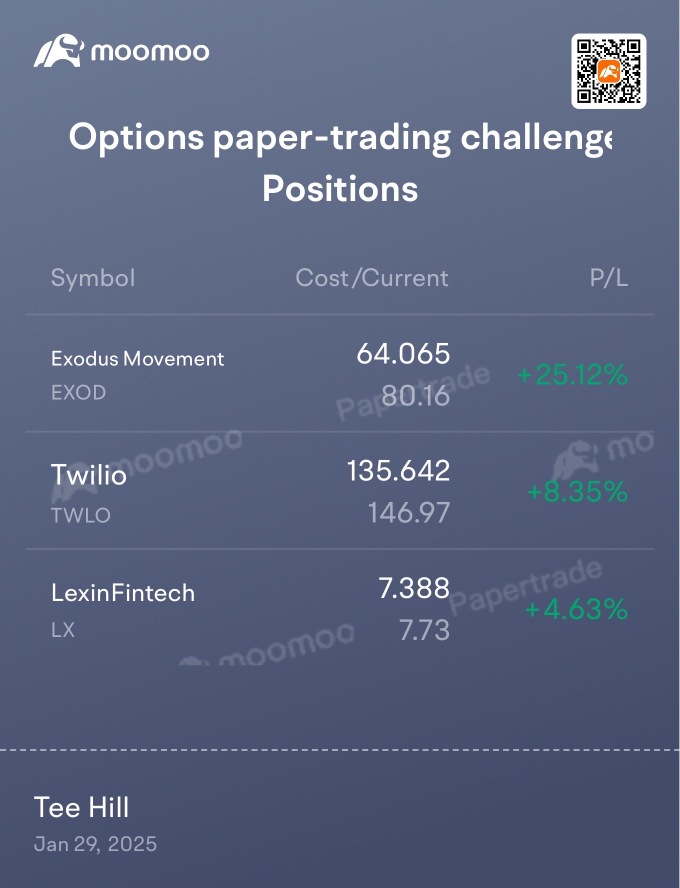
LX LexinFintech
- 10.530
- +1.460+16.10%
- 10.410
- -0.120-1.14%
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Capital Trend
No Data
News
Express News | Shares of US-listed Chinese Companies Are Trading Higher on Renewed Optimism Over Policy Stimulus as Chinese Economic Policymakers Are Set to Gather on Monday to Discuss Measures to Boost Consumption
LexinFintech (LX.US) will release its Earnings Reports after the market closes on March 18.
$LexinFintech (LX.US)$ will release the Earnings Report after the market close on March 18, and investors should pay attention. How was the previous performance? $LexinFintech (LX.US)$ Q3 2024 revenue is 3.662 billion yuan, Net income is 0.31 billion yuan, EPS is 1.84 yuan. Q4 2023 revenue is 3.509 billion yuan, Net income is 12.097 million yuan, EPS is 0.08 yuan. The accounting standards used for the data above are US_GAAP. Futubull reminds: 1. There is no strict regulation on the fiscal year division for companies listed in Hong Kong and the US; it's entirely up to the company to decide, hence each Earnings Report.
Hong Kong stock movement | Qianxin Bio-B (02509) surged over 30% at the end of trading. LexinFintech's new indication supplemental application was approved, and the application for Crohn's disease indication has been accepted.
At the end of trading, Tsang Wing Biotechnology-B (02509) surged over 30%, with a rise of 30.03% to 11.3 Hong Kong dollars and a transaction amount of 3.8883 million Hong Kong dollars.
Express News | Shares of US-listed Chinese Companies Are Trading Lower Amid Global Investor Concerns Regarding the Trump Administration's Trade Policies and China's Retaliatory Tariffs on US Farm Goods, Which Took Effect Monday
LexinFintech Holdings Ltd. to Report Fourth Quarter and Fiscal Year 2024 Unaudited Financial Results on March 18, 2025
Asian Equities Traded in the US as American Depositary Receipts Lower in Thursday Trading
Comments